They close, we open!
Time runs fast, but science runs even faster. It seems like yesterday when young Werner Forssmann performed the first human cardiac catheterization: he anesthetized his own lower arm, inserted a 66-cm-long ureteric catheter into his antecubital vein, threaded it partly before walking some distance to the X-ray department, and then advanced it into his right ventricle under the guidance of a fluoroscope. Thus, invasive cardiology was born in 1929.
THE LAST DECADES
Since then the field of invasive cardiology has grown consistently, following the development of new, feasible and effective procedures, such as transcatheter aortic valve implantation, percutaneous mitral and tricuspid valve repair, left atrial appendage closure, percutaneous ventricular restoration, and the treatment of various congenital heart diseases. The topic of the day deals with a novel, attractive, and percutaneous procedure which will surprise you and may raise interesting points for discussion.
ATRIAL SEPTAL DEFECT (ASD)
I am sure you are familiar with atrial septal defects and resulting shunts, as well as the precise indications on how and when these defects should be closed according to the guidelines. How would you respond if I told you that there also is a procedure that does just the opposite: it creates a hole in the heart? The procedure is known as balloon atrio-septostomy.
OLD STUFF
Balloon atrio-septostomy is not new. It has been used in cyanotic congenital heart disease to increase interatrial mixing of blood. A balloon is used to tear the interatrial septum.
POTENTIAL NEW INDICATION
But maybe this procedure where an opening is created in the interatrial septum can also be used for other purposes? One of these is heart failure patients with preserved ejection fraction. We at the medical University of Vienna are now participating in the multicenter REDUCE LAP-HF trial (REDUCE Elevated Left Atrial Pressure in Patients with Heart Failure) whose purpose is to evaluate exactly this subject. The small controlled opening is created with the help of an investigational device, the interatrial septal device (IASD) system II, which is shown below. The device is available exclusively for clinical investigations outside the U.S.

Interatrial Septal Device (IASD) System II.
The device is intended to create and maintain a persistent small left-to-right shunt. The latter would potentially reduce left atrial pressure, thus reducing pulmonary edema and dyspnea, and potentially improving quality of life in HFpEF patients. Box 1 shows you the criteria according to which patients can be enrolled for the trial:
Key inclusion and exclusion criteria for the trial:
Inclusion criteria:
1. Left ventricular ejection fraction (LVEF) estimated by echocardiography ≥ 40%
Exclusion criteria:
1. Presence of significant valve disease defined by echocardiography as:
- Mitral valve regurgitation >moderate
- Tricuspid valve regurgitation ≥moderate
- Aortic regurgitation or aortic stenosis ≥moderate
2. Hypertrophic obstructive cardiomyopathy, restrictive cardiomyopathy, constrictive pericarditis, cardiac amyloidosis or other infiltrative cardiomyopathy (e.g. hemochromatosis, sarcoidosis)
3. Right ventricular dysfunction, defined as:
- More than mild right ventricular dysfunction as determined by transthoracic echocardiography (TTE): OR
- TAPSE - RV volume ≥ LV volume.
4. Echocardiographic evidence of intra-cardiac mass, thrombus, or vegetation.
OUR PATIENT
Our patient, 71 years old and suffering from exertional dyspnea (NYHA functional class III), is the first HFpEF patient to be implanted in Vienna. He was a non-smoker and his lung function was normal. Our patient’s heart was quite difficult to image by transthoracic echo (TTE) because of a poor acoustic window. His left ventricle (LV) was severely hypertrophic, non-dilated, and its pump function was preserved (his LVEF was 63%). His left and right atria were severely enlarged, but the size and function of the right ventricle were normal. With regard to the valves, only mild mitral, and mild tricuspid regurgitation were noted.
TRANSESOPHAGEAL ECHOCARDIOGRAPHIC (TEE) FINDINGS
Our patient’s heart looked like this on TEE:
Mid-esophageal 4-chamber view (0°) showing a normal-sized LV with preserved pump function and
mild mitral regurgitation.
Focusing on the atrial septum: Do you see anything abnormal?
Mid-esophageal bicaval view and its perpendicular view obtained by the 3Dsimultaneous multiplane mode.
This 3D modepermits the use of a dual screen to simultaneously display two real-time images.
The first image s typically a reference view of a particular structure (here the interatrial septum) while the second image shows an orthogonal view.
It was dumbbell-like and hyperechogenic, indicating lipomatous hypertrophy.
THE PROCEDURE STEP BY STEP
The procedure was performed under fluoroscopic and TEE guidance. A standard transseptal puncture was followed by balloon atrioseptostomy; A balloon was then placed across the interatrial septum and inflated.
Mid-esophageal bicaval view (90° to 110°) displaying the balloon inflated across the interatrial septum.
When the balloon was retrieved, a left-to-right shunt was clearly visible, consistent with the elevated left atrial pressure.
Mid-esophageal bicaval view (90° to 110°) showing the left-to-right shunt.
HERE COMES THE IASD SYSTEM II
The delivery system was then advanced across the septum, following a wire placed with its tip in the left upper pulmonary vein. The delivery system was then introduced over the wire. The 3D loop shown below displays the tip of the delivery system, exactly where the device is mounted.
Real-time 3D zoom mode; left atrial view of the interatrial septum. The tip of the delivery system is inside the left atrium.
When the tip of the system was positioned in the left atrium, the left side of the device was deployed - as shown below.
Real-time 3D zoom mode; left atrial view of the interatrial septum. The left atrial side of the device is deployed.
The delivery system and device was then retracted towards and against the left side of the septum.
Real-time 3D zoom mode; left atrial view of the interatrial septum. The majority of the device makes good contact with the septum.
THE END OF THE PROCEDURE
The device barely fit into the thin (primum septum) part of the interatrial septum without becoming distorted by the lipomatous part of the interatrial septum. Now it was time to release the right atrial side of the device and retrieve the delivery system. Finally the guide wire is withdrawn. The 3D image below shows the final result. A perfect result: Excellent contact between the septum and the device.
Real-time 3D zoom mode; left atrial view of the interatrial septum. The device is perfectly in place.
And here is the left-to-right interatrial shunt with which our patient went home.
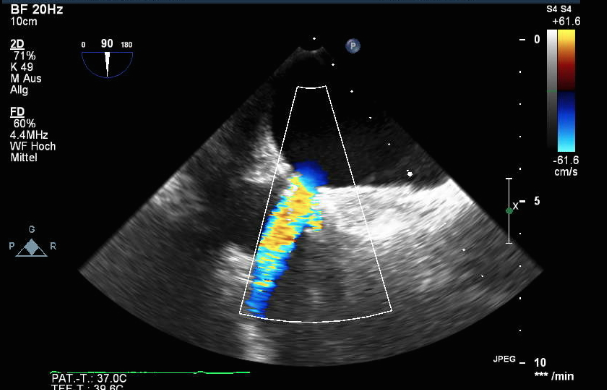
Mid-esophageal bicaval view (90° to 110°) showing the final result.
YOUR OPINIONS
Undoubtedly a cool, simple procedure. Subsequent follow-up over the next three years will give us information about the hemodynamic sequelae and whether our patient is doing better. In the meantime, we are curious to know what you think. How do you treat heart failure patients with elevated left atrial pressure when appropriate medical management does not help?
Post your comments and remember: progress is born out of new investigation and intuition. Open up your minds… you could be the Werner Forssmann of tomorrow!
The reduce LAP Team, from left to right:
Front row: Lynne Ford, Irene Lang, Anna Maria Pistritto, Hajra Barucic
Middle row: Diana Murer, Mario Gerges, Stefan Bruner, Thomas Binder, Christian Gerges
Top row: Martin Tomsich, Harald Gabriel
Not in the picture, to be mentioned: Mary de Jesus, Marion Thiel-Hitmann
Best,
Anna